Smart coating for orthopaedic implants
Smart coatings for surgical orthopaedic implants are being proposed that can monitor strain on devices to provide early warning of implant failures, while killing infection-causing bacteria.
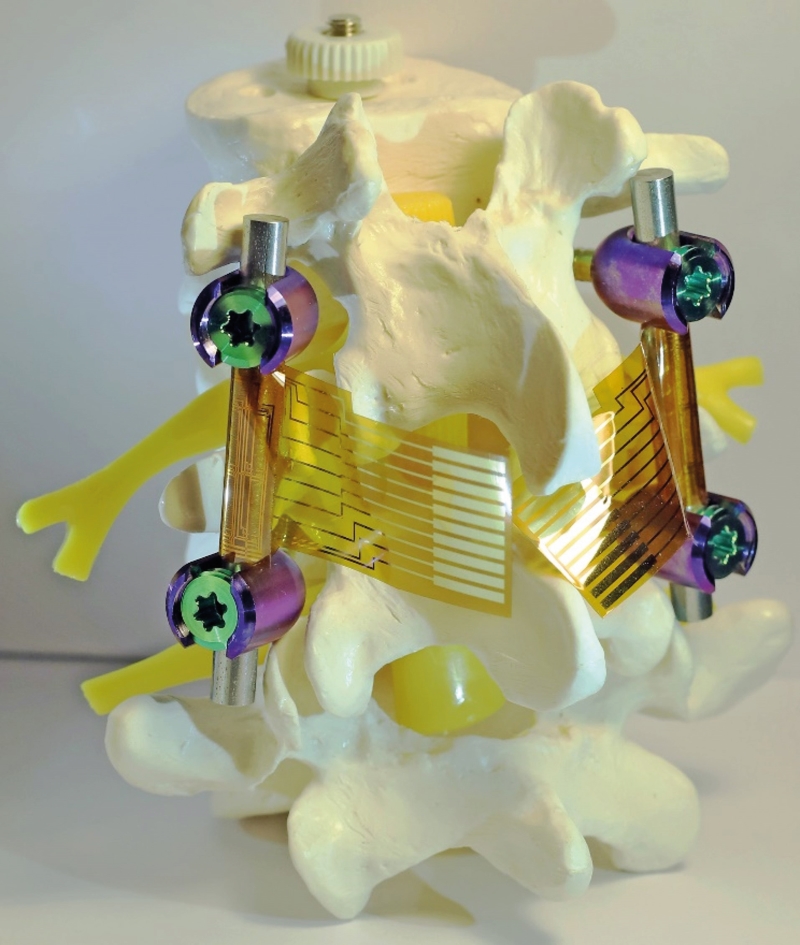
The coatings, from researchers at the University of Illinois Urbana-Champaign, USA, integrate flexible sensors with a nanostructured antibacterial surface inspired by the wings of dragonflies and cicadas.
Both infection and device failure are major problems with orthopaedic implants, each affecting up to 10% of patients, notes Associate Professor Qing Cao at the University.
The existing technique to prevent such infections is to load antibiotics onto the surface of the implant and let them release slowly to kill the bacteria in the vicinity. However, it can cause drug resistance and incur toxic side-effects to human cells, tissues and organs.
Additionally, it is difficult is to monitor the healing progress and detect instrument failures. Currently, this is achieved by imaging (e.g. CT scan). However, the radiologic investigations only give indirect evidence with limited detectivity and specificity, say the researchers. Moreover, they are expensive and expose patients to radiation risks.
The team has developed a dual-functional smart coating polymer foil to address both problems simultaneously. The flexible foil coating can be directly applied onto the curved surfaces of the implants.
On its outer surface, they have fabricated a high density of nanopillar arrays to mimic the surface structure of dragonfly wings. These nanopillars can kill any bacteria that attaches on top by puncturing through the cell walls.
Cao says, 'It is a mechanical interaction without directly releasing any chemicals, and it will not affect mammalian (e.g. human) cells.
'On its inner surface intended for conformal contact with the implant, we fabricate an array of strain sensors to monitor the stresses applied on the implant in real time.'
Cao adds, 'Since the normal strain for implants is very low, we built our strain sensors based on high-quality single-crystalline silicon…to achieve the required sensitivity.
'Although single-crystalline silicon is usually rigid and brittle, by reducing its thickness to ~100nm, i.e. one thousandth the width of a human hair, the silicon films become flexible enough to be applied on the curved surfaces of implants.
'Electronic control circuits were built on such ultra-thin silicon membranes at the same time to enable mapping of the strain distribution over the entire surface of the implant.'
In the new method, Cao says the bacterial load in the in vivo trials has been reduced by more than 100 times compared to planar control without relying on any chemicals. The nanopillars can kill more than 99% of pathogenic bacteria in vitro (both gram positive and gram negative types).
While a strain change on the implant below 0.01% was accurately detected without modifying the internal structure of the implant. Cao says both results are 'unprecedented'.
'The films can successfully prevent infection in a mouse without incurring any toxic side-effects or acute inflammatory response. The bacteria-killing nanostructures and the strain-mapping electronics were well preserved in vivo for at least eight weeks.'
They will test the functionality and efficacy of the smart coating foils in large animal models to move a step closer to clinical trials.