‘Metal whispering’ to recycle e-waste
A process coined ‘metal whispering’ is recovering pure and precious metals from alloys in electrical waste using oxygen at relatively low temperatures.
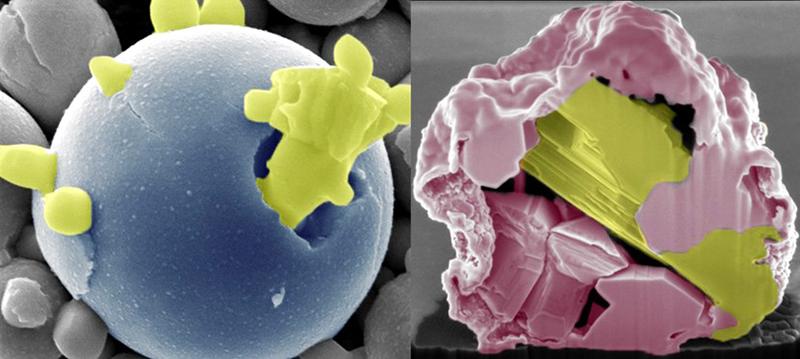
The technology’s name comes from its ability to control exactly how and where alloy components fall apart, or de-alloy.
Engineers at Iowa State University, USA, say they can tune oxidation by temperature, oxidant partial pressure, time and composition to de-alloy a metal by gradually moving the most reactive components to the surface where they form stalagmite-like spikes of metal oxides. The technology works at lower temperatures (between 2600C and 3700C) and without use of corrosive chemical leaching methods.
Research Lead Martin Thou explains that this effect is achieved using the differences in oxidation potential to draw the most reactive component (e.g. a rare earth) to the surface as an oxide, while driving the less oxidising components to the core of the alloy.
‘This process leads to two different states, one as an oxide – mechanically brittle and lower density – and [one] a pure metal(s),’ he says. ‘By selective de-alloying, residual metals demix in their pure form either in the core (those that contract on cooling e.g. gold or silver) or as spiky fingering on the surface of the oxide (those that expand on solidifying e.g. bismuth).’
Component recovery is then achieved either through density, mechanical or solubility differences.
‘What we demonstrate here is that the traditional electrochemical or high-temperature methods may not be necessary in metal purification as the metal’s reactivity can be used to drive separation,’ he adds.
‘The ability to exploit intrinsic properties of the metal is key in developing efficient, environmentally benign recycling or upcycling methods. Since we are not using large-scale equipment, this method is amenable to local small-scale recycling for target metals.’
Testing is still underway – tin alloys have been found the most amenable to demonstrate the concept. ‘We will be discussing other alloy systems in follow-up publications. We also liked bismuth because it expands when it solidifies and could be used to illustrate the role of thermal expansion coefficient in the process,’ Thou suggests.
The new method is still under exploration to understand the extent of its capability. ‘The obvious [future application] is in e-waste recycling or upcycling, but it can also be applied in alloy purification, protection of reactive metals by forming a protective oxide shell, among others, that we are still exploring.’







