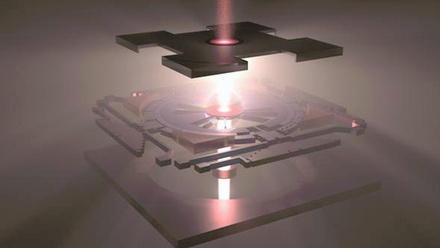
Membrane aids lithium extraction from salt lakes
A crystalline carbon-nitride membrane is being heralded as a potential game-changer for lithium extraction from salt lakes.

The study at the Chinese Academy of Sciences introduces a 'congener-welded' membrane that reportedly outperforms traditional polymer membranes.
The design mimics biological ion channels, with reportedly remarkable efficiency and durability in separating lithium ions from magnesium ions in salt-lake brine.
The membrane structure combines crystalline and amorphous forms of polymer carbon nitride. This provides pore uniformity and narrowness so that the membrane can exclude larger hydrated magnesium ions, while allowing for smooth lithium-ion transport, similar to barrier-free ion transport in natural ion channels.
'Such a phenomenon attributes to the synergistic effect of size sieving of crystalline carbon nitride channels and the ion-channel interaction. When entering the channel, the binding number for Mg2+ to the nitride site was smaller than the lost binding with hydration shell, meaning Mg2+ experiences a certain barrier, while the transport for Li+ was almost barrier-less,' Professor Jian Liu explains.
To make the membrane, the team loads crystalline carbon nitride on an anode aluminium oxide substrate by vacuum filtration. Amorphous carbon nitride is then deposited on the crystalline layer surface via thermal deposition. This continuous and close-contact interface occurs due to their homologous properties, forming a unique structure.
The membrane is said to achieve a selectivity ratio of 1,708 for the extraction of highly dilute lithium ions (0.002M) from concentrated magnesium ions (1.0M) – which the team says is crucial to address prevalent high magnesium content in various lithium sources.
So, for 1L of solution, after 24 hours of permeation, the lithium-ion content is 1,708 times that of Mg2+.
Conventionally, obtaining a 0.002M solution from 1M lithium solution would require 500 times dilution by adding a lithium chloride solid into a certain volume of water.
Liu continues, 'The most useful and challenging task is to sieve dilute Li+ out of concentrated Mg2+, which highlights the superiority and prospect of our membrane thanks to its high Li/Mg selectivity ratio.
'Being coupled with a multi-pass process, the membrane may perform surprisingly. But for practical application, more cost-effective strategies need to be developed for scalable preparation of membrane to handle the brine volume from large-scale operations, which has not been involved in the current work.'
The dual functionality opens up possibilities beyond lithium extraction, reflects the team, such as seawater desalination, wastewater treatment and gas separation.
They also think it could be used for energy conversion, such as salinity energy harvesting. Surprisingly, the membrane may realise nano-confined catalysis, by taking advantage of the nano-channel and photo-responsiveness of crystalline carbon nitride.