Cleaning up healthcare - a circular economy for medical devices
How can materials support a circular economy in medical devices?
Healthcare needs to clean up its act. This critical industry cares for our populations but is simultaneously one of the major contributors to global carbon emissions, a major consumer of natural resources and produces ever-growing waste streams.
Considering this stark picture, it is unsurprising that the UN prioritised the development of climate-resilient and low-carbon sustainable health systems as part of the UN Framework Convention on Climate Change. Introduced at COP26, the framework requests that health systems commit to moving towards net-zero emissions, ideally by 2050.
In response, the UK’s Greener NHS programme has set ambitious targets to reach carbon net-zero by 2045, with the European Green Deal setting similar ambitions. Clearly, there is a need and political impetus to reduce the environmental burden of healthcare, considering both carbon emissions and resource use.
Tackling this situation is complex, but the concept of the circular economy (CE) can provide a robust solution. A heavy reliance on single-use products and associated packaging – particularly within surgical settings – means medical devices are one of the most significant contributors to carbon emissions in healthcare.
Initially catalysed by concerns about the infection risk from reusing instrumentation, single-use medical devices have since become entrenched through commoditisation and market forces.
Medical products are manufactured from raw materials, transported and used before being passed into clinical waste streams, which preclude recycling and typically mandate incineration.
In short, a linear economy dominates. While it is possible to make environmental improvements within this linear framework, such as by optimising designs in favour of lightweight components, it fundamentally limits the scope of what can be achieved. Ultimately, we need a different model that avoids the waste of natural resources.
Closing the loop
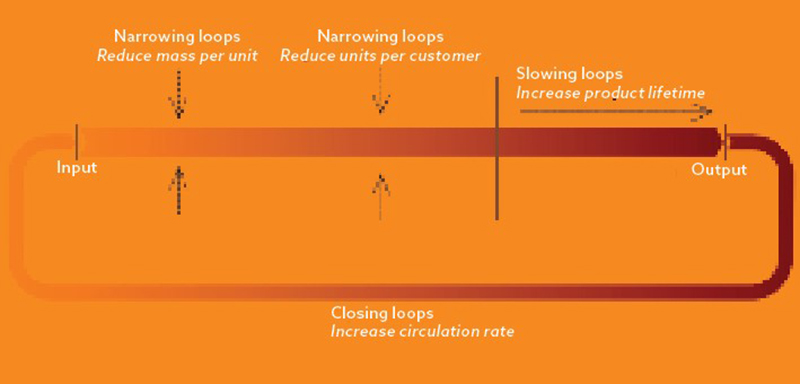
The central tenet of CE is to reduce or eliminate waste by closing resource loops to conserve material resources in their highest possible value states. Put simply, where possible, we must move from single-use medical devices to reusable models, as shown in the image opposite.
In a circular approach, recycling is considered the last resort before consigning resources to waste. The direct reuse of a device after cleaning and sterilisation – its highest value state – is far preferable, or alternatively after a form of reprocessing, such as sharpening blunted scissor blades.
In addition to closing resource loops, CE encourages ‘narrowing loops’ by using fewer resources to produce a device, such as through innovations in packaging. It also promotes ‘slowing loops’ – increasing device longevity, such as by using coatings/surface technology. These CE principles provide a simple but powerful framework to guide improvements in medical device design.
While they focus primarily on the flow of resources, improving the circularity of a medical device is also generally associated with lower-carbon emissions.
Materials are central to establishing CE in medical devices. As part of UK Research and Innovation’s ReMed project, we are exploring how using materials and materials technology can support and stimulate the move toward circularity.
In the wash
Cleaning and sterilisation is a key challenge in making medical devices suitable for reuse. To close the loop, the materials and assemblies must be robust to these processes. This is most stringent with sterile devices such as for surgical instrumentation.
The process involves pre-cleaning and decontamination to remove biomatter before sterilisation. Autoclave (high-pressure steam) is then the established sterilisation method in most health services. The high temperature, pressure and moisture involved can cause significant degradation in materials, such as blunting sharp edges on surgical instruments and increasing susceptibility to corrosion. In addition, autoclaves are often employed for convenience, even if a device needs lower-level disinfection.
Applying circular principles, there is an opportunity to design medical devices for use with alternative sterilisation methods, such as UV-C, gamma, hydrogen peroxide and ethylene oxide. These are less harsh on materials, extending longevity, and have substantially lower resource and carbon footprints than energy-intensive autoclaves.
For example, a study published in the Journal of Hospital Infection on Shedding a light on ultraviolet-C technologies in the hospital environment highlights UV-C as an effective means to disinfect ultrasound probes. It provides a fast, low-power process that causes negligible material degradation compared to autoclave, extending the device lifespan.
Design and material selection are also key factors when considering how a medical device can survive multiple cycles of use. Design for cleaning and sterilisation must be part of the process, rather than assuming a de facto use of autoclave.
This should also encompass functional aspects, such as internal lumens or complex jaw assemblies in surgical graspers, and non-functional design features that may preclude otherwise appropriate methods. UV-C requires line-of-sight exposure, so enclosed lumens or occluded surfaces are not compatible, but hydrogen peroxide may provide an alternative.
Alternative cleaning and sterilisation methods, coupled with appropriate design and materials, open the opportunity for new approaches to device design that close resource loops.
Slowing the loop
The medical sector is highly regulated, particularly in terms of supply chains and the composition of medical-grade materials. In this risk-averse environment, manufacturers naturally tend towards established materials, such as stainless steels or medical-grade polymers, with well-understood mechanical and biocompatibility properties. However, surface treatments can bridge the gap and provide scope for innovation, enhancing device functionality and longevity. Two leading types of surface treatment are those based on ceramics and polymers.
In metal devices, contact with tissue and body fluid causes the release of metallic particles and can cause toxicity. When repetitively in contact with chloride ions, from bodily fluids or cleaning detergent, even stainless steels are susceptible to localised corrosion.
Diamond-like carbon (DLC) has been popular since the early 1990s in areas like surgery, to prolong an instrument’s life and prevent metallic particle release.
The coatings exhibit inertness, improved cracking, wear resistance, corrosion resistance and reduced friction.
DLC also shows antibacterial properties against Staphylococcus aureus, Klebsiella pneumonia and Pseudomonas aeruginosa. These properties are attributed to low-surface energy, with minimum molecular adhesion, and can be increased further by adding fluorine or silver, as reported in a 2008 study on the Reduction of bacterial adhesion on modified DLC coatings in Colloids and Surfaces B: Biointerfaces.
Zirconium oxide is another promising coating. Also known as zirconia, it has low-thermal conductivity compared to most metals, making it an excellent protection layer in extreme applications, such as for thermal barrier coatings in aircraft and marine engines. It is also effective in preserving the durability of surgical instruments.
Zirconia also provides other attractive properties, including high fracture toughness and wear resistance, while possessing a similar modulus of elasticity to stainless steel. This makes it appealing as a surface treatment to increase the longevity of existing stainless-steel instrumentation.
Polymer coatings provide various opportunities to tackle biofouling – the build-up of microorganisms, bacteria and biomolecules on surfaces that impair device function. In medical devices, biofouling is often caused by the deposition of proteins from blood or bodily fluids, risking patient cross-contamination.
The process of biofouling is associated with surface wettability. Material modification typically focuses on changing this property, and strategies include the use of hydrophilic surfaces, zwitterionic coatings and superhydrophobic surfaces.
Hydrophilic coatings employ hydrophilic polymer chains that act like brushes to repel contaminants like proteins. Examples include polyethylene glycol, poly (2-hydroxyethyl methacrylate), polyvinylpyrrolidone and poly(hydroxypropyl methacrylamide).
Zwitterionic coatings comprise an equal balance of anions and cations, providing a hydrated neutral-charge surface that precludes protein attachment.
Promising zwitterionic coatings include polycarboxybetaine (PCB), betaine methacrylate sulphonate (SBMA), betaine carboxylate methyl methacrylate (CBMA) and 2-methacryloyloxyethyl phosphorylcholine (MPC).
Bioinspiration has led to superhydrophobic surfaces inspired by the lotus leaf, which have acquired wide recognition in medical applications. For example, in a 2022 study titled Bioinspired ultra-low fouling coatings on medical devices to prevent device-associated infections and thrombosis in J Colloid Interface Sci, patterned surfaces using hydrophobic polydimethylsiloxane (PDMS) showed effective anti-thrombogenic properties and excellent corrosion barriers against aggressive ions.
While coating technologies promote circularity, the additional cost and resource use they entail must be balanced against the benefits they realise. Similarly, their commercial readiness and ability to be applied at scale must be considered. However, existing examples already highlight that coatings can be commercially viable and clinically advantageous.
World Precision Instruments’ microscissors use a ‘DiaMED’ DLC coating to increase durability and minimise biomatter adherence to ease cleaning and decontamination. In the context, it is also interesting to consider that surface coatings could be used as ‘sacrificial layers’, which can be replenished as part of a reprocessing stage, reducing the need for remanufacture.
Narrowing the loop
Medical waste poses a real barrier to achieving circularity in healthcare systems. A 2018 WHO report on Health-care waste, reported that 75-90% is considered ‘general waste’, and half of this results from packaging of medical devices.
Even if there is the potential for recycling, in practice, it is challenging to extract and separate these packaging materials at scale from clinical waste streams, so they are destined for incineration.
Packaging in medical devices is subject to higher regulatory standards. It must meet stringent criteria in which materials should provide a microbial barrier, be non-toxic, allow sterilisation, provide direct visibility for visual inspection (i.e., transparency), and maintain an extended shelf life.
Devices are often ‘double-packed’ to ensure sterility. This could be avoided in many cases – rationalising packaging solutions to reduce their volume and thus narrow the resource loop.
Bioplastics could provide a more circular alternative source from non-fossil fuels. They diversify feedstocks to renewable sources, with options including mycelium mushrooms, corn, sugarcane and seaweed. However, plant-based bioplastics are resource-intensive and may compete with food resources.
In contrast, polyhydroxyalkanoates (PHA) are produced as the metabolites of microorganisms. With lower costs and carbon footprint compared to plant-based solutions, the PHA market is forecasted for rapid growth. Further exploration of alternative materials in packaging is needed to help make them commercially viable (where margins are particularly tight).

A bioplastic film made from polyhydroxyalkanoates
© Adapted from T. Palmeiro-Sanchez, V. O’Flaherty, P.N.L. Lens, (2022) Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future, J Biotechnol 348 10-25. doi.org/10.1016/j.jbiotec.2022.03.001. creativecommons.org/licenses/by/4.0/ with permission from ElsevierHowever, all these packaging solutions are typically developed as a single-use commodity. Finding truly circular solutions requires the development of reusable packaging.
For example, rigid aluminium containers reduced carbon emissions by 85% compared to single-use wraps, as published in a Sustainability article on Reducing the environmental impact of sterilization packaging for surgical instruments in the operating room: A comparative life cycle assessment of disposable versus reusable systems. Further investigation into materials and solutions will make this more prevalent – reducing materials consumption and narrowing the loop.
Taking stock
Medical devices form a significant part of the challenge to reduce the environmental impact of the healthcare industry. The current reliance on single-use systems and disposable packaging requires a fundamentally different approach to achieve strict climate targets.
The CE provides a powerful framework that can guide and catalyse the efficient use of materials. In essence, we should seek to reuse – thus minimising resource consumption and generating associated emissions.
Material research is central in moving towards a CE for medical devices. The sector needs continued innovation to address the barriers and challenges associated with the practical implementation of new materials and coatings.
For example, can supply chains meet demand? Do they meet the regulatory requirements? Are they commercially viable, and do functional benefits justify design changes in established products?
To address these challanges, engagement and encouragement from all stakeholders to catalyse positive change are essential.
Regulatory bodies must incentivise rather than penalise innovation, which aims to lower environmental impact. The medical device industry must embrace potentially disruptive changes to combat inertia. Healthcare systems should ensure procurement processes evaluate environmental impact and costs based on device lifetime and not point of purchase.
By collaborating, the healthcare industry can clean up its act – ensuring materials innovation drives a move towards a CE in medical devices.








